Patterns and mechanisms of sediment charging and discharging driven by groundwater level fluctuations
-
摘要:
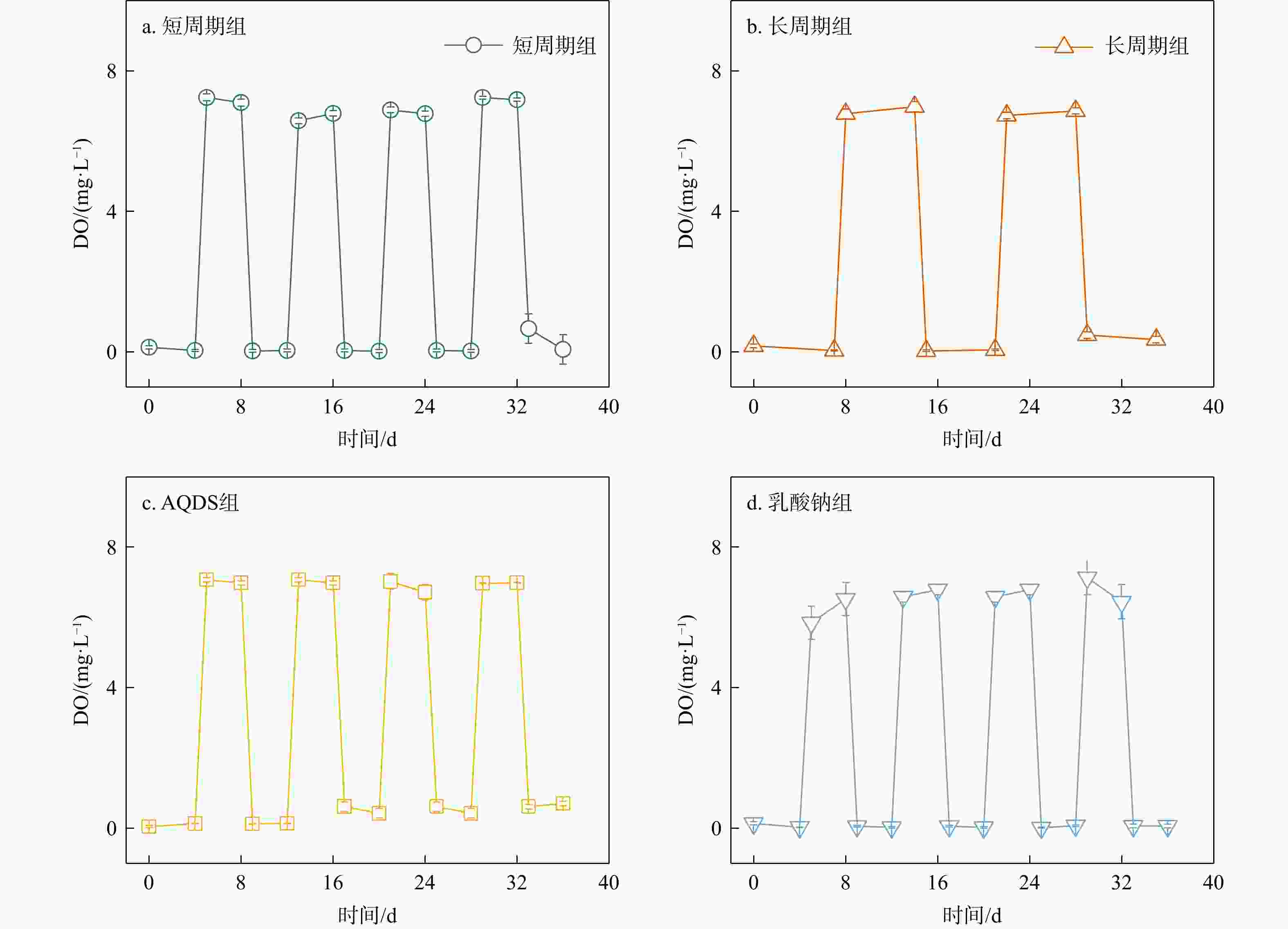
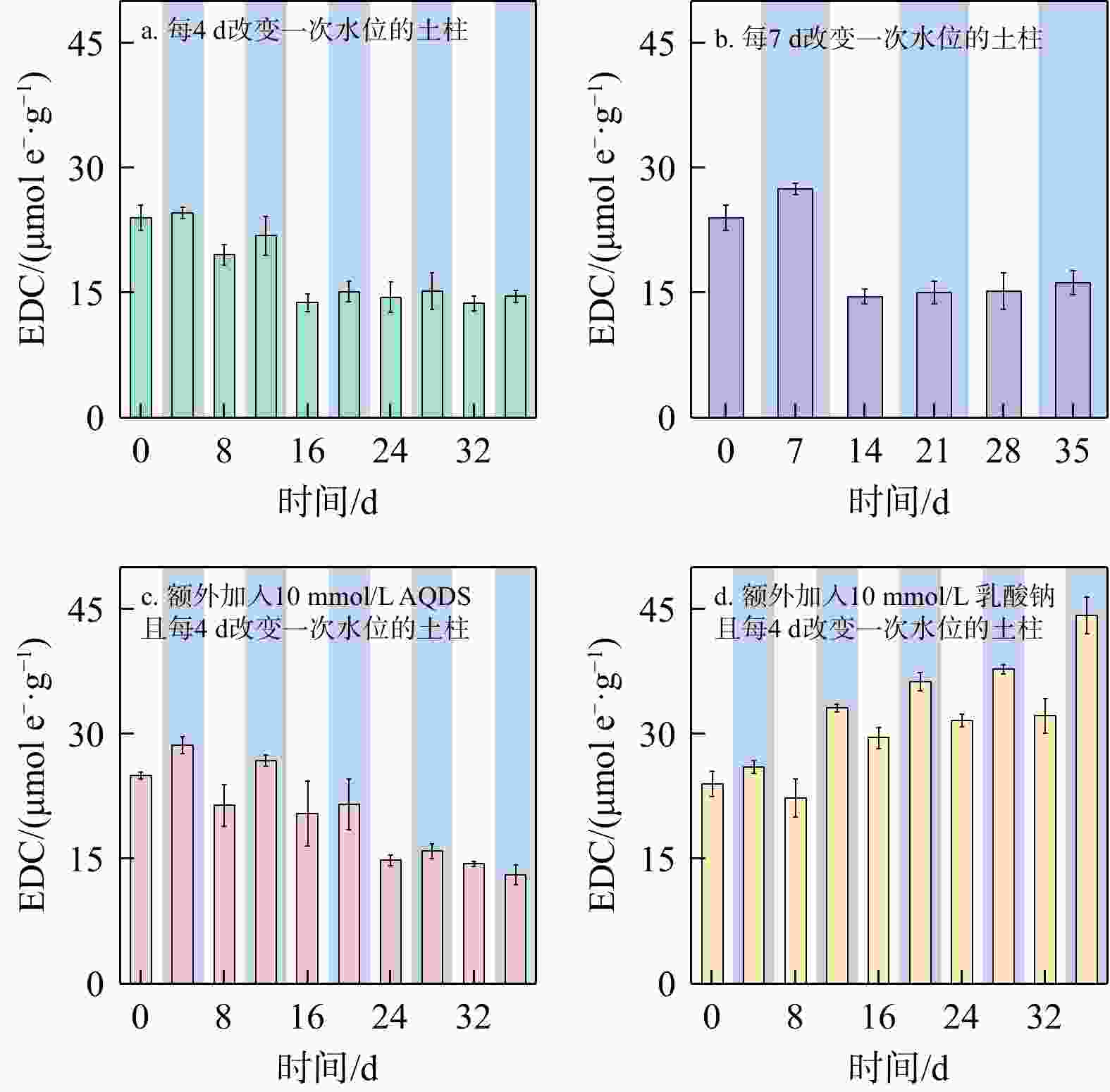
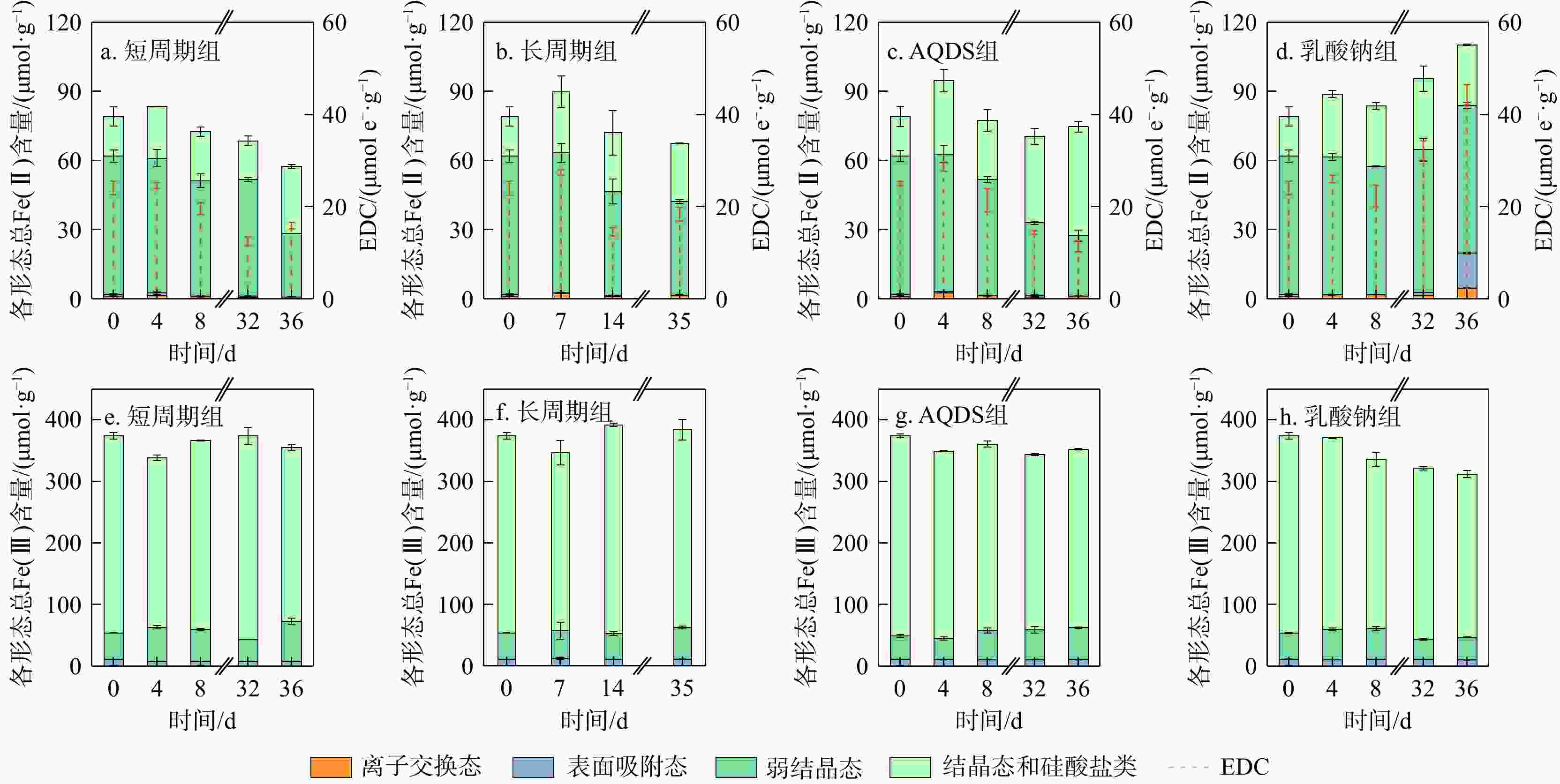
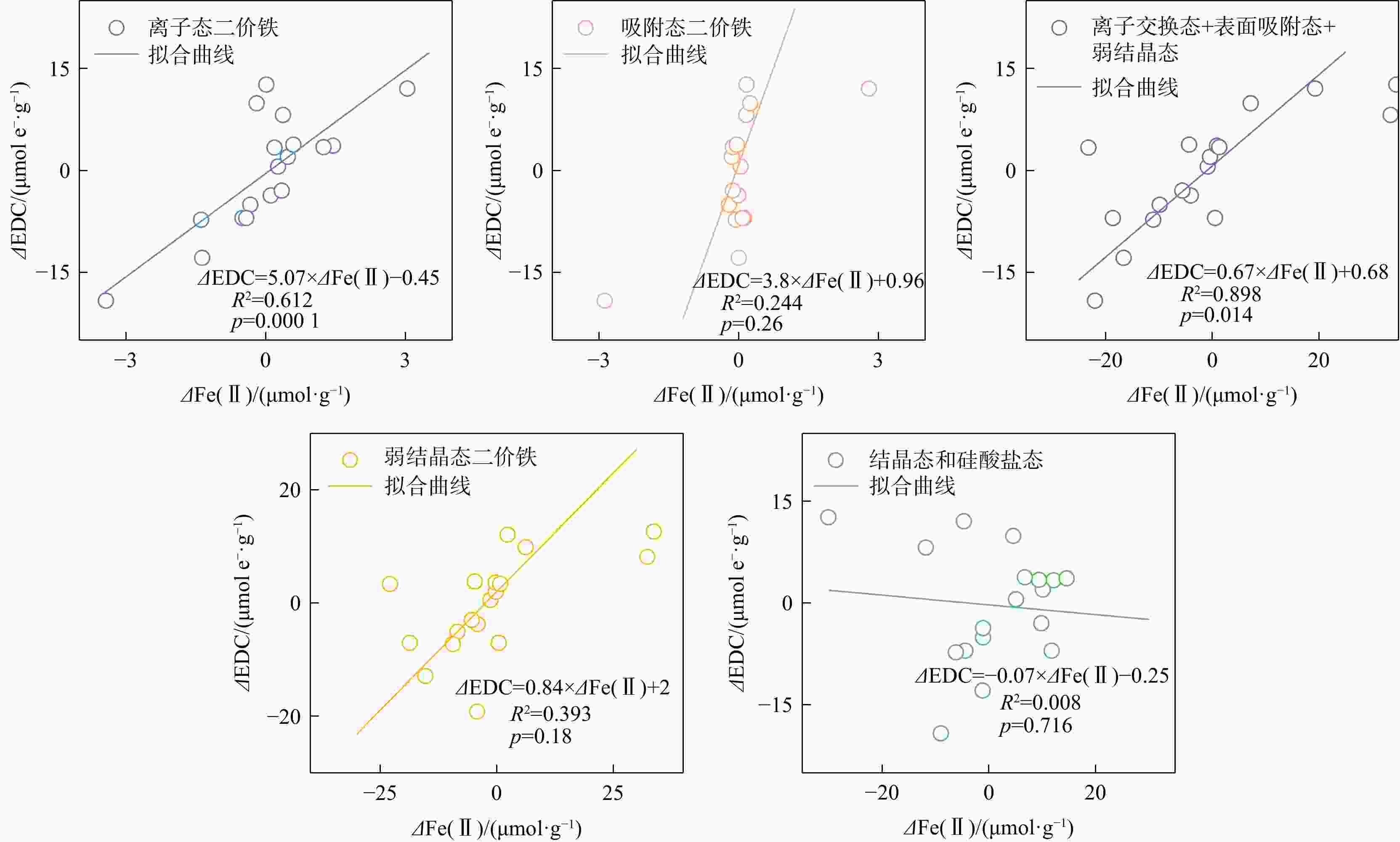
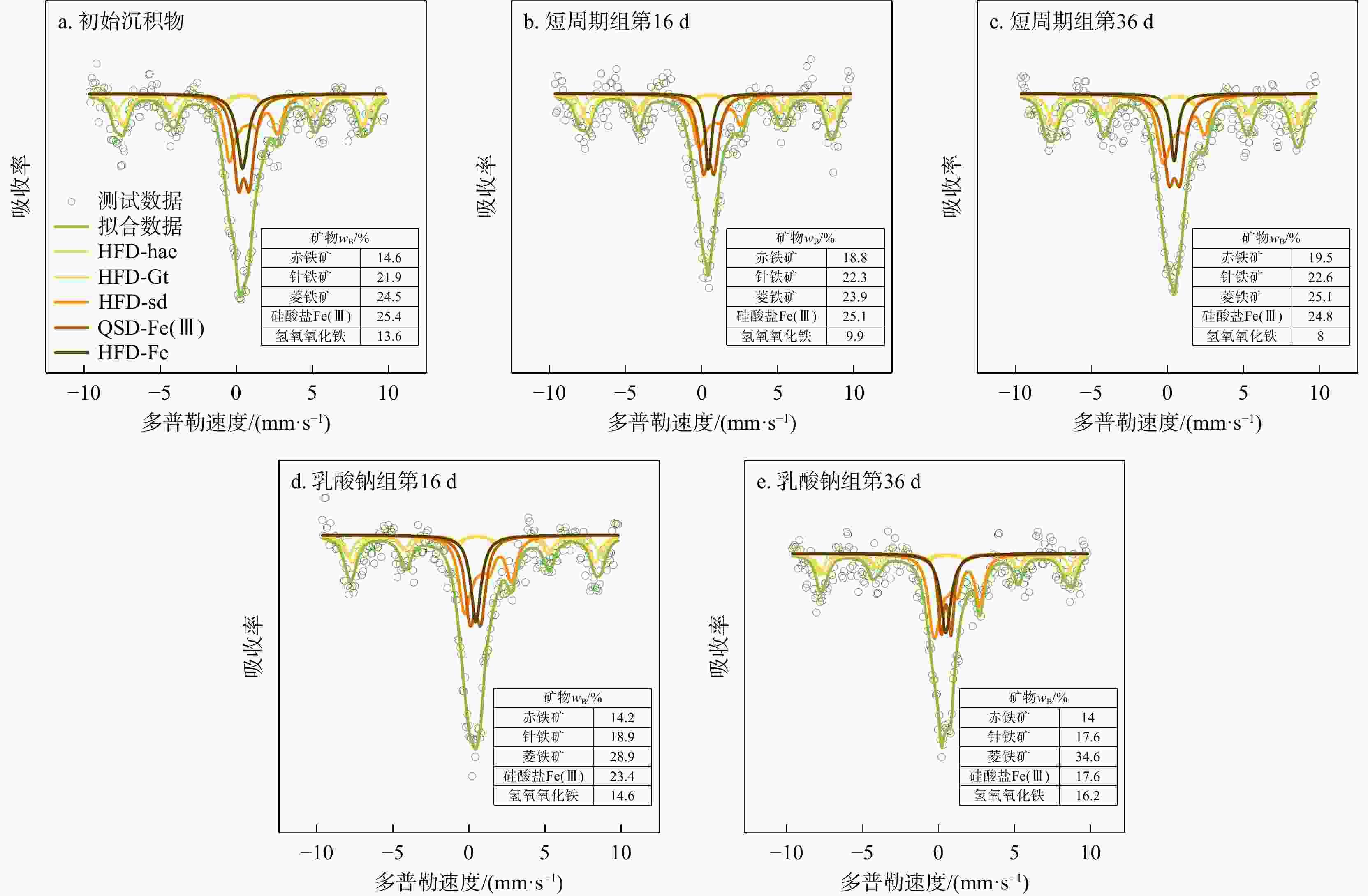
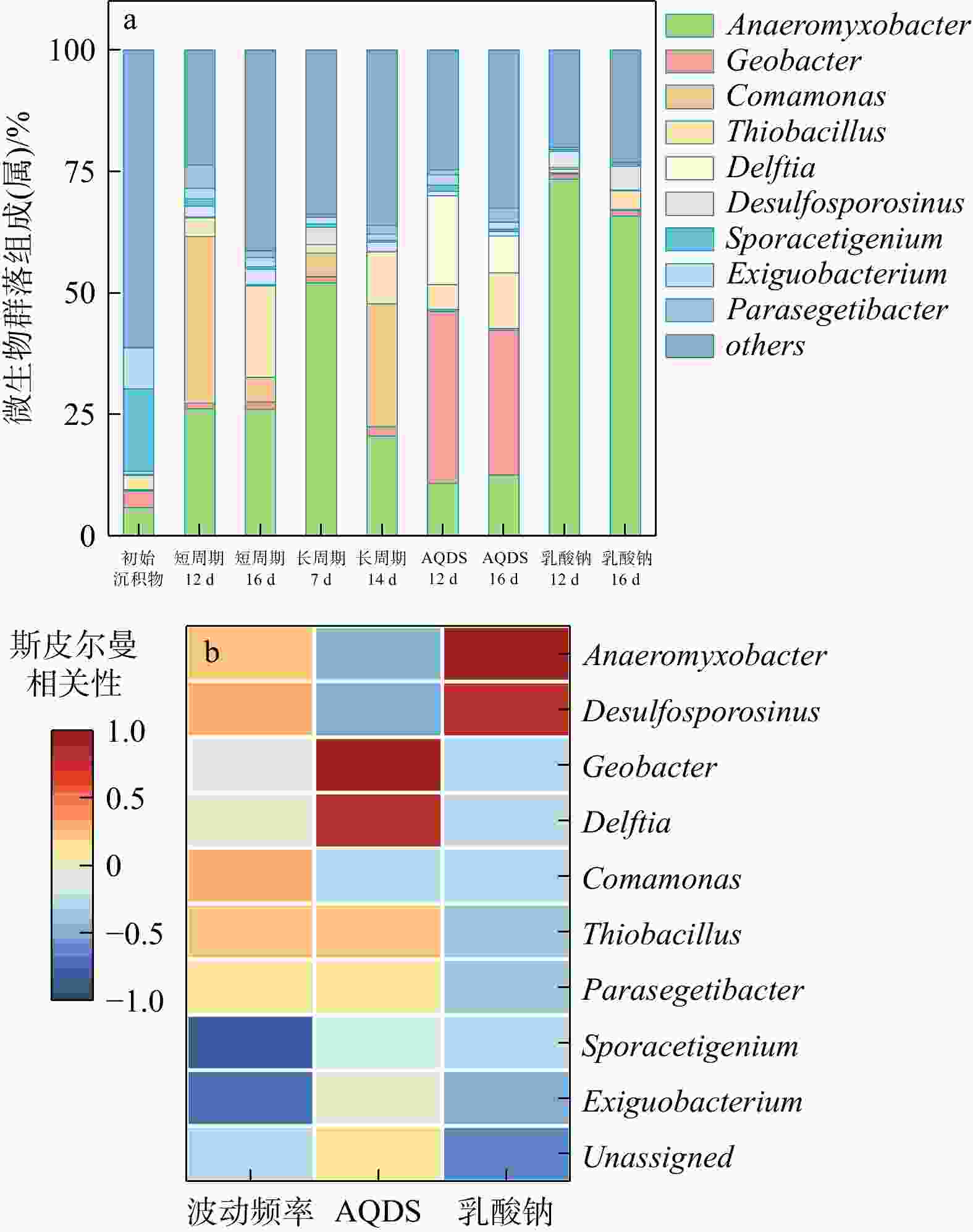
地下环境中生物地球化学反应的本质是电子转移。沉积物是地下环境中重要的电子储库,在水位波动驱动下可进行循环的电子储存与释放,显著影响地下环境中的物质转化和元素循环。然而,目前对于地下水位波动驱动的沉积物充放电规律及机制仍缺乏认识。本研究构建了一维土柱体系模拟地下水位波动带,结合化学分析、铁矿物形态分析和分子生物学技术,探究了水位波动驱动的沉积物充放电规律与机制。结果表明,在短周期波动模式下,沉积物可以完成2次充−放电循环,最大充放电量分别为2.3,8 μmol e−·g−1,最大充放电速率为0.577,2.012 μmol e−·g−1·d−1。沉积物的给电子容量(EDC)主要由吸附态、离子交换态和高活性弱结晶态Fe(Ⅱ)贡献。水位波动通过驱动Fe(Ⅲ)生物还原−Fe(Ⅱ)化学氧化实现沉积物的充放电循环。随着还原−氧化反应的循环,铁氧化物的生物可利用性降低,导致沉积物无法持续充放电。电子穿梭体蒽醌-2,6-二磺酸(AQDS)的输入初期显著提高了充放电速率,但加速了Fe(Ⅲ)生物可利用性的降低,最终使充放电速率逐渐下降,充放电循环在第3周期停止。电子供体乳酸钠的输入显著富集了铁还原菌
Anaeromyxobacter ,维持了Fe(Ⅲ)的高生物可利用性,使沉积物的充放电速率显著提高,在水位波动下能进行持续的充放电循环。本研究揭示了不同水位波动条件下沉积物充放电行为的变化规律及其控制机制,为地下水污染防控提供了新的策略。Abstract:Objective Electron transfer is fundamental to biogeochemical reactions in subsurface environments. Sediments act as key electron reservoirs capable of cyclic electron storage and release under groundwater level fluctuations, thereby significantly influencing material transformation and elemental cycling. However, the patterns and mechanisms governing sediment charging and discharging driven by groundwater level fluctuations remain poorly understood.
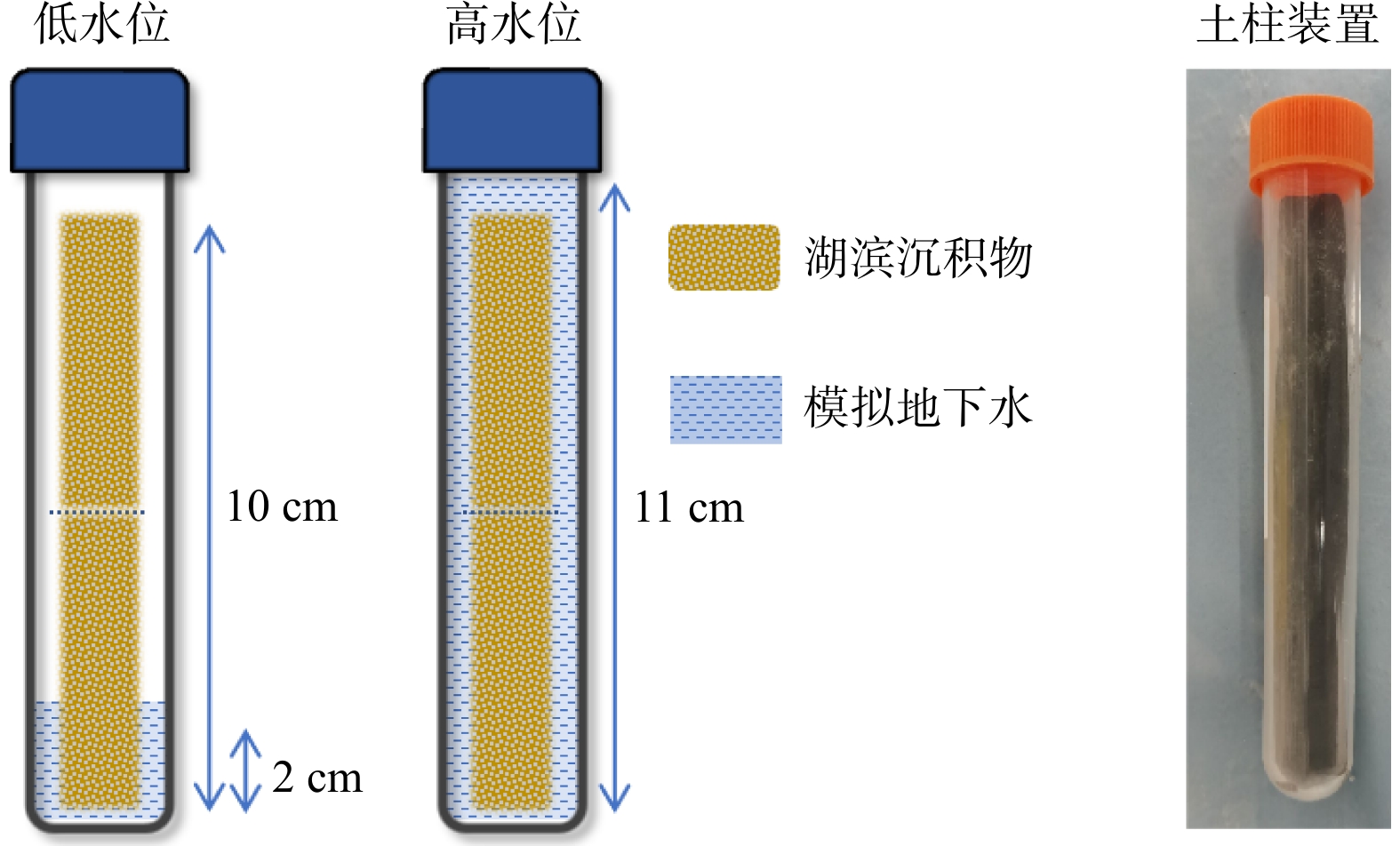
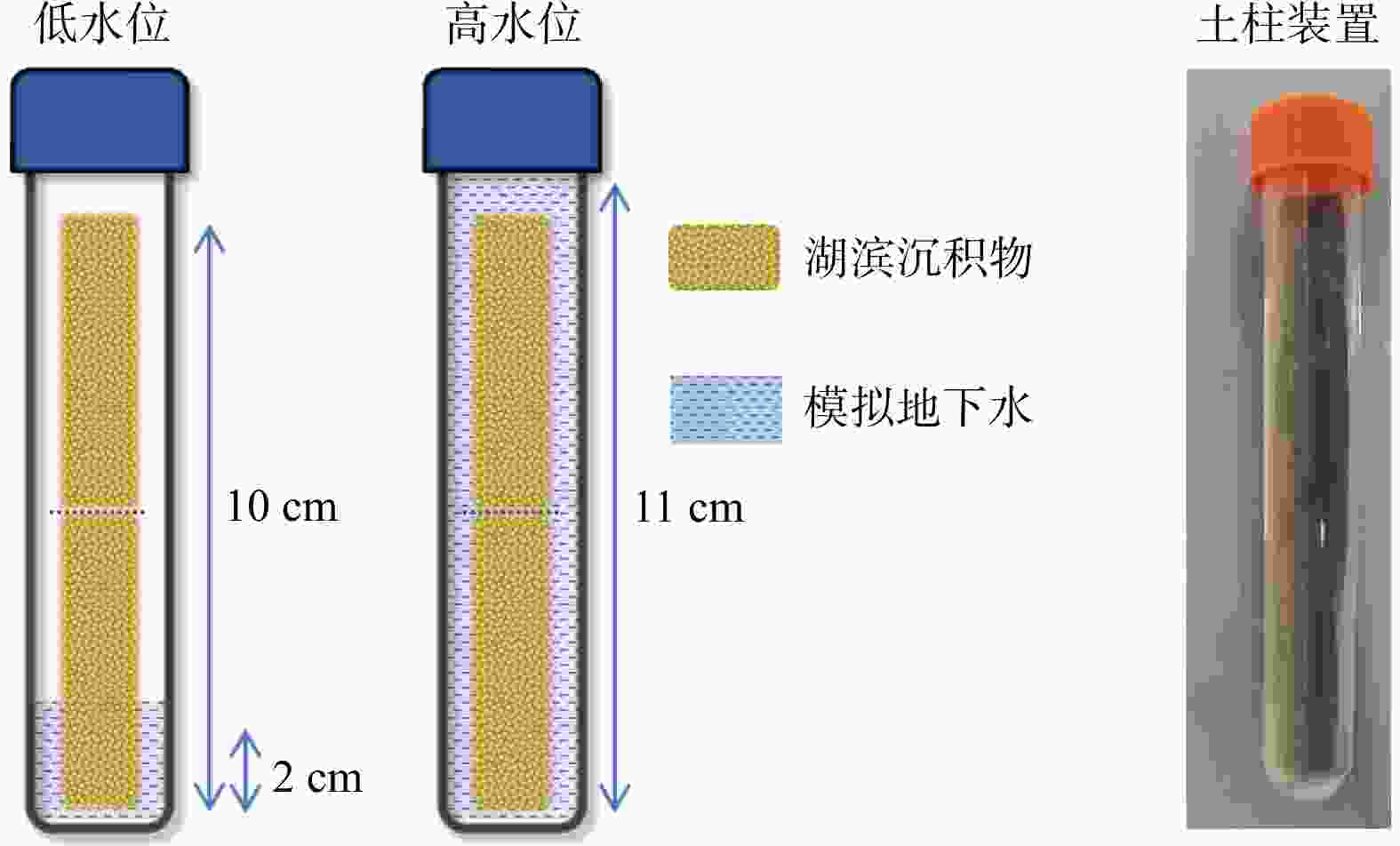
Methods In this study, a one-dimensional soil column system was developed to simulate the groundwater fluctuation zone. The patterns and mechanisms of sediment charging and discharging driven by groundwater level fluctuations were investigated using a combination of chemical analyses, iron mineral speciation, and molecular biological techniques.
Results The results showed that under short-cycle fluctuation conditions, sediments completed two charging-discharging cycles, with maximum charge/discharge capacities of 2.3, 8 μmol e−·g−1, and peak rates of 0.577, 2.012 μmol e−·g−1·d−1, respectively. The electron donating capacity (EDC) of the sediments was mainly contributed by adsorbed, ion-exchangeable, and highly reactive weakly crystalline Fe(Ⅱ). Water level fluctuations drove microbial Fe(Ⅲ) reduction followed by chemical Fe(Ⅱ) oxidation, thereby enabling sediment charging and discharging cycles. However, repeated redox cycles reduced the bioavailability of iron oxides, ultimately hindering sustained electron storage and release. The introduction of the electron shuttle anthraquinone-2,6-disulfonate (AQDS) significantly increased the initial charging and discharging rate but also accelerated the decline in Fe(Ⅲ) bioavailability, resulting in a gradual decrease in the charging and discharging rate and the cessation of cycling after the third cycle. In contrast, the addition of sodium lactate, as an electron donor, significantly enriched the iron-reducing bacterium
Anaeromyxobacter , maintained high Fe(Ⅲ) bioavailability, and markedly enhanced the charging and discharging rate, supporting sustained cycling under water level fluctuations.Conclusion This study reveals the variation patterns and regulatory mechanisms of sediment charging and discharging behaviors under different groundwater level fluctuation regimes, providing new strategies for groundwater pollution prevention and control.
-
Key words:
- groundwater level fluctuation /
- sediment /
- iron cycling /
- electron transfer /
- biogeochemical reaction
-
-
[1] LIU Y C, FEI Y H, MENG S H, et al. Hydrochemical evolution of groundwater and soils in the water-level-fluctuation zone[J]. Environmental Earth Sciences, 2019, 78(22): 647. doi: 10.1007/s12665-019-8660-y [2] PENTRÁKOVÁ L, SU K, PENTRÁK M, et al. A review of microbial redox interactions with structural Fe in clay minerals[J]. Clay Minerals, 2013, 48(3): 543-560. doi: 10.1180/claymin.2013.048.3.10 [3] WU W X, HUANG C H, TANG Z R, et al. Response of electron transfer capacity of humic substances to soil microenvironment[J]. Environmental Research, 2022, 213: 113504. doi: 10.1016/j.envres.2022.113504 [4] KLÜPFEL L, PIEPENBROCK A, KAPPLER A, et al. Humic substances as fully regenerable electron acceptors in recurrently anoxic environments[J]. Nature Geoscience, 2014, 7(3): 195-200. doi: 10.1038/ngeo2084 [5] BYRNE J M, KLUEGLEIN N, PEARCE C, et al. Redox cycling of Fe(2) and Fe(3) in magnetite by Fe-metabolizing bacteria[J]. Science, 2015, 347(6229): 1473-1476. doi: 10.1126/science.aaa4834 [6] ZHOU Z, HENKEL S, KASTEN S, et al. The iron “redox battery” in sandy sediments: Its impact on organic matter remineralization and phosphorus cycling[J]. Science of The Total Environment, 2023, 865: 161168. doi: 10.1016/j.scitotenv.2022.161168 [7] 贾卓鹏, 毕俊擘, 原勇, 等. 基于LM-BPNN的高寒地区地下水微生物-毒理指标预测模型[J]. 地质科技通报, 2025(2): 368-377. doi: 10.19509/j.cnki.dzkq.tb20240345JIA Z P, BI J B, YUAN Y, et al. Prediction model of groundwater microbiological toxicological indicators in alpine regions based on LM-BPNN[J]. Bulletin of Geological Science and Technology, 2025(2): 368-377. (in Chinese with English abstract doi: 10.19509/j.cnki.dzkq.tb20240345 [8] 李杰彪, 周志超, 郭永海, 等. 高放废物处置北山预选区地下水化学形成机制模拟研究[J]. 地质科技通报, 2025(4): 340-353. doi: 10.19509/j.cnki.dzkq.tb20240194LI J B, ZHOU Z C, GUO Y H, et al. Hydrogeochemical modeling of groundwater formation mechanism at the Beishan preselected site for high-level radioactive waste disposal[J]. Bulletin of Geological Science and Technology, 2025(4): 340-353. (in Chinese with English abstract doi: 10.19509/j.cnki.dzkq.tb20240194 [9] FARNSWORTH C E, VOEGELIN A, HERING J G. Manganese oxidation induced by water table fluctuations in a sand column[J]. Environmental Science & Technology, 2012, 46(1): 277-284. [10] LI X X, SHENG A X, DING Y F, et al. A model towards understanding stabilities and crystallization pathways of iron (oxyhydr)oxides in redox-dynamic environments[J]. Geochimica et Cosmochimica Acta, 2022, 336: 92-103. doi: 10.1016/j.gca.2022.09.002 [11] PEIFFER S, KAPPLER A, HADERLEIN S B, et al. A biogeochemical-hydrological framework for the role of redox-active compounds in aquatic systems[J]. Nature Geoscience, 2021, 14(5): 264-272. doi: 10.1038/s41561-021-00742-z [12] AEPPLI M, KAEGI R, KRETZSCHMAR R, et al. Electrochemical analysis of changes in iron oxide reducibility during abiotic ferrihydrite transformation into goethite and magnetite[J]. Environmental Science & Technology, 2019, 53(7): 3568-3578. [13] CHEN C M, DONG Y J, THOMPSON A. Electron transfer, atom exchange, and transformation of iron minerals in soils: The influence of soil organic matter[J]. Environmental Science & Technology, 2023, 57(29): 10696-10707. [14] LEE S Y, ROH Y, KOH D C. Oxidation and reduction of redox-sensitive elements in the presence of humic substances in subsurface environments: A review[J]. Chemosphere, 2019, 220: 86-97. doi: 10.1016/j.chemosphere.2018.11.143 [15] QIAN A, LU Y X, ZHANG Y T, et al. Mechanistic insight into electron transfer from Fe(2)-bearing clay minerals to Fe (hydr)oxides[J]. Environmental Science & Technology, 2023, 57(21): 8015-8025. [16] YUAN Y, ZHANG H, WEI Y Q, et al. Onsite quantifying electron donating capacity of dissolved organic matter[J]. Science of The Total Environment, 2019, 662: 57-64. doi: 10.1016/j.scitotenv.2019.01.178 [17] WANG F, CHE R X, DENG Y C, et al. Air-drying and long time preservation of soil do not significantly impact microbial community composition and structure[J]. Soil Biology and Biochemistry, 2021, 157: 108238. doi: 10.1016/j.soilbio.2021.108238 [18] ZHANG Y T, TONG M, LU Y X, et al. Directional long-distance electron transfer from reduced to oxidized zones in the subsurface[J]. Nature Communications, 2024, 15: 6576. doi: 10.1038/s41467-024-50974-x [19] ZHANG L, LIU Y F, JIN M G, et al. Influence of seasonal water-level fluctuations on depth-dependent microbial nitrogen transformation and greenhouse gas fluxes in the riparian zone[J]. Journal of Hydrology, 2023, 622: 129676. doi: 10.1016/j.jhydrol.2023.129676 [20] 张鹏, 卢钰茜, 袁松虎. 一种快速检测非均质含水层介质氧化还原容量的方法[P]. 中国专利: CN118583847A 2024-09-03.ZHANG P, LU Y X, YUAN S H. Method for rapidly detecting redox capacity of heterogeneous aquifer medium [P].China patent: CN118583847A 2024-09-03. (in Chinese) [21] LI S, KAPPLER A, ZHU Y G, et al. Mediated electrochemical analysis as emerging tool to unravel links between microbial redox cycling of natural organic matter and anoxic nitrogen cycling[J]. Earth-Science Reviews, 2020, 208: 103281. doi: 10.1016/j.earscirev.2020.103281 [22] YIN X, HUA H, DYER J, et al. Assessing reactive iron mineral coatings in redox transition zones with sequential extraction[J]. ACS Earth and Space Chemistry, 2022, 6(2): 368-379. doi: 10.1021/acsearthspacechem.1c00352 [23] ZHANG P, LIU J Y, YU H, et al. Kinetic models for hydroxyl radical production and contaminant removal during soil/sediment oxygenation[J]. Water Research, 2023, 240: 120071. doi: 10.1016/j.watres.2023.120071 [24] XIE W J, YUAN S H, TONG M, et al. Contaminant degradation by •OH during sediment oxygenation: Dependence on Fe(2) species[J]. Environmental Science & Technology, 2020, 54(5): 2975-2984. [25] RANCOURT D G, PING J Y. Voigt-based methods for arbitrary-shape static hyperfine parameter distributions in Mössbauer spectroscopy[J]. Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, 1991, 58(1): 85-97. [26] CHEN C M, KUKKADAPU R K, LAZAREVA O, et al. Solid-phase Fe speciation along the vertical redox gradients in floodplains using XAS and mössbauer spectroscopies[J]. Environmental Science & Technology, 2017, 51(14): 7903-7912. [27] HONTY M, FREDERICKX L, BANERJEE D, et al. Fe distribution, redox state and electrochemical activity in Boom Clay[J]. Applied Geochemistry, 2021, 125: 104857. doi: 10.1016/j.apgeochem.2020.104857 [28] GORSKI C A, AESCHBACHER M, SOLTERMANN D, et al. Redox properties of structural Fe in clay minerals. 1. electrochemical quantification of electron-donating and-accepting capacities of smectites[J]. Environmental Science & Technology, 2012, 46(17): 9360-9368. [29] LIU J, SHENG A X, LI X X, et al. Understanding the importance of labile Fe(3) during Fe(2)-catalyzed transformation of metastable iron oxyhydroxides[J]. Environmental Science & Technology, 2022, 56(6): 3801-3811. [30] LI Y, ZHANG C Q, YANG M J, et al. Carbonate accelerated transformation of ferrihydrite in the presence of phosphate[J]. Geoderma, 2022, 417: 115811. doi: 10.1016/j.geoderma.2022.115811 [31] ZHANG Y T, TONG M, YUAN S H, et al. Interplay between iron species transformation and hydroxyl radicals production in soils and sediments during anoxic-oxic cycles[J]. Geoderma, 2020, 370: 114351. doi: 10.1016/j.geoderma.2020.114351 [32] ZACHARA J M, KUKKADAPU R K, PERETYAZHKO T, et al. The mineralogic transformation of ferrihydrite induced by heterogeneous reaction with bioreduced anthraquinone disulfonate (AQDS) and the role of phosphate[J]. Geochimica et Cosmochimica Acta, 2011, 75(21): 6330-6349. doi: 10.1016/j.gca.2011.06.030 [33] COKER V S, BELL A M T, PEARCE C I, et al. Time-resolved synchrotron powder X-ray diffraction study of magnetite formation by the Fe(3)-reducing bacterium Geobacter sulfurreducens[J]. American Mineralogist, 2008, 93(4): 540-547. doi: 10.2138/am.2008.2467 [34] 张千帆, 曾强, 刘邓, 等. 腐殖酸对微生物还原绿脱石结构Fe(Ⅲ)的促进作用[J]. 地质科技情报, 2016, 35(6): 205-211.ZHANG Q F, ZENG Q, LIU D, et al. Enhancement of microbial reduction of structural Fe(Ⅲ)in nontronite by humic acid[J]. Geological Science and Technology Information, 2016, 35(6): 205-211. (in Chinese with English abstract [35] LOVLEY D R, HOLMES D E, NEVIN K P. Dissimilatory Fe(3) and Mn(4) reduction[M]. Advances in Microbial Physiology, Academic Press, 2004: 49, 219-286. [36] WANG K, JIA R, LI L N, et al. Community structure of Anaeromyxobacter in Fe(3) reducing enriched cultures of paddy soils[J]. Journal of Soils and Sediments, 2020, 20(3): 1621-1631. doi: 10.1007/s11368-019-02529-7 [37] LI H Q, LI H, ZHOU X Y, et al. Distinct patterns of abundant and rare subcommunities in paddy soil during wetting-drying cycles[J]. Science of The Total Environment, 2021, 785: 147298. doi: 10.1016/j.scitotenv.2021.147298 [38] Succession of metabolically active Anaeromyxobacter community in flooded paddy soil owing to organic carbon input[J]. Soil Science Society of America Journal, 2022, 86(5): 1169-1181. [39] DANTAS J M, MORGADO L, CATARINO T, et al. Evidence for interaction between the triheme cytochrome PpcA from Geobacter sulfurreducens and anthrahydroquinone-2, 6-disulfonate, an analog of the redox active components of humic substances[J]. Biochimica et Biophysica Acta (BBA) - Bioenergetics, 2014, 1837(6): 750-760. doi: 10.1016/j.bbabio.2014.02.004 [40] SUN W M, KRUMINS V, DONG Y R, et al. A combination of stable isotope probing, illumina sequencing, and co-occurrence network to investigate thermophilic acetate- and lactate-utilizing bacteria[J]. Microbial Ecology, 2018, 75(1): 113-122. doi: 10.1007/s00248-017-1017-8 [41] ZHU C. Variation of Anaeromyxobacter community structure and abundance in paddy soil slurry over flooding time[J]. African Journal of Agricultural Reseearch, 2011, 6(28): 6107-6118. -



 下载:
下载: