In situ pH measurement and prediction modelling of the impure CO2-water system under high-temperature and high-pressure conditions
-
摘要:
含CO2的气体注入到深部含水层中会溶解形成碳酸,导致含水层的pH值下降,进而造成矿物的溶解或沉淀,影响CO2地质利用与封存的安全性和有效性。pH值作为能表征溶液化学性质的重要参数,因此通过实验测量纯/非纯CO2饱和溶液体系的pH值,并结合模型预测可以评估CO2地质封存条件下的化学变化。在原位条件下通过电势法和光谱法测量了温度范围35~93℃、压力范围0.38~18 MPa时,纯CO2-H2O体系和非纯CO2-H2O体系的pH值;并建立了基于溶解度校准的组分化学平衡模型,对纯/非纯CO2-H2O体系的pH值进行了计算和预测评价。结果表明:N2和CH4均会对CO2饱和体系产生影响,使CO2在水中的溶解度降低,pH值增大,且CH4的影响大于N2。模型能较好地进行纯CO2-水体系的pH值预测,最大偏差不超过0.05个pH;在非纯CO2-H2O体系中有一定的偏差,主要为50℃以及CO2和杂质气体比例为1∶9的条件下,偏差在0.15个pH值以内。电势法和光谱法能进行高温高压条件下的原位pH值测量,纯/非纯CO2-H2O体系的pH预测模型具有较好的准确性,本文的研究成果为非纯CO2注入地层产生的化学变化提供了理论参考,对提高碳封存的安全性和有效性具有重要意义。
Abstract:The dissolution of CO2-containing gases into deep aquifers results in the formation of carbonic acid, which lowers the pH value of the aquifer and may cause the dissolution or precipitation of minerals, thereby affecting the safety and effectiveness of CO2 geological utilization and storage.
Objective pH is a key parameter for characterizing the chemical properties of a solution. Experimental measurement and model prediction of the pH values of pure and impure CO2-saturated systems can be used to evaluate the chemical changes under CO2 geological storage conditions.
Methods In this study, the pH values of pure and impure CO2-water systems were measured in situ using potentiometric and spectroscopic methods, under temperatures conditions ranging from 35 to 93℃ and pressure condictions raging from 0.38 to 18 MPa. A component chemical equilibrium model calibrated using solubility data, was developed to calculate and predict the pH values of pure and impure CO2-water systems.
Results The results show that that N2 and CH4 influence the CO2-saturated systems by reducing CO2 solubility in water and increasing the pH value, with CH4 having a greater effect than N2. The model can accurately predict the pH values of pure CO2-water systems, with a maximum deviation within 0.05 pH units. In impure CO2-water systems, deviations are mainly observed under the condition of 50℃ and a CO2-to-impurity gas ratio of 1∶9, with deviations within 0.15 pH units.
Conclusion The potentiometric and spectroscopic methods are applicable for in situ pH measurements under high-temperature and high-pressure conditions, and the pH prediction model for pure and impure CO2-water systems shows good accuracy. The findings provide theoretical support for understanding the chemical changes induced by the injection of impure CO2 into geological formations, which is of great significance for enhancing the safety and effectiveness of carbon storage.
-
Key words:
- impure CO2 /
- CO2-saturated system /
- impure CO2-water system /
- in situ pH measurement /
- pH prediction
-
表 1 不同温度下溴酚蓝指示剂的$ p{K}' $和$ {e}_{1} $、$ {e}_{2} $、$ {e}_{3} $
Table 1. Measured $ p{K}' $ and $ {e}_{1} $,$ {e}_{2} $,$ {e}_{3} $ of bromophenol blue at different temperatures
表 2 原位条件下纯/非纯CO2−水饱和体系的pH测量结果
Table 2. Measured pH for pure/impure CO2-water saturated systems under in situ conditions
方法 体系 总压力/MPa CO2分压/MPa 35℃ pH 总压力/MPa CO2分压/MPa 50℃ pH 总压力/MPa CO2分压/MPa 70℃pH 电
势
法CO2-H2O 0.38 0.38 3.66 0.36 0.36 3.70 0.37 0.37 3.79 0.60 0.60 3.59 0.61 0.61 3.62 0.60 0.60 3.73 1.01 1.01 3.48 1.00 1.00 3.48 1.01 1.01 3.56 2.43 2.43 3.35 2.42 2.42 3.34 2.42 2.42 3.40 6.28 6.28 3.21 6.28 6.28 3.22 6.28 6.28 3.25 CO2-N2-H2O
(1∶1)0.79 0.40 3.62 0.77 0.39 3.68 0.78 0.39 3.88 1.20 0.60 3.58 1.18 0.59 3.62 1.20 0.60 3.60 2.00 1.00 3.47 2.01 1.01 3.50 2.01 1.01 3.53 4.79 2.40 3.37 4.80 2.40 3.39 4.80 2.40 3.45 12.01 6.01 3.24 12.00 6.00 3.27 12.00 6.00 3.36 方法 体系 总压力/MPa CO2分压/MPa 50℃ pH 总压力/MPa CO2分压/MPa 75℃ pH 总压力/MPa CO2分压/MPa 93℃ pH 光
谱
法CO2-H2O 0.38 0.38 3.75 0.38 0.38 3.93 0.38 0.38 3.98 0.60 0.60 3.61 0.60 0.60 3.71 0.60 0.60 3.86 1.00 1.00 3.55 1.00 1.00 3.65 1.00 1.00 3.69 2.42 2.42 3.36 2.42 2.42 3.41 2.42 2.42 3.47 6.30 6.30 3.17 6.30 6.30 3.21 6.30 6.30 3.24 9.00 9.00 3.13 9.00 9 3.17 9 9 3.23 12.00 12.00 3.11 12 12 3.15 12 12 3.19 15.00 15.00 3.10 15 15 3.12 15 15 3.14 18.00 18.00 3.08 18 18 3.10 18 18 3.13 CO2-N2-H2O
(1∶1)0.76 0.38 3.76 0.76 0.38 3.80 0.76 0.38 3.89 1.20 0.60 3.68 1.20 0.60 3.69 1.20 0.60 3.78 2.00 1.00 3.59 2.00 1.0 3.59 2.00 1.00 3.69 4.80 2.40 3.36 4.80 2.40 3.45 4.80 2.40 3.50 12.00 6.00 3.25 12.00 6.00 3.28 12.00 6.00 3.37 CO2-CH4-H2O
(1∶1)0.76 0.38 3.78 0.76 0.38 3.83 0.76 0.38 3.87 1.20 0.60 3.69 1.20 0.60 3.75 1.20 0.60 3.79 2.00 1.00 3.59 2.00 1.00 3.62 2.00 1.00 3.69 4.80 2.40 3.48 4.80 2.40 3.48 4.80 2.40 3.53 12.00 6.00 3.32 12.00 6.00 3.35 12.00 6.00 3.39 CO2-N2-H2O
(3∶7)1.30 0.39 3.78 1.30 0.39 3.80 1.30 0.39 3.91 2.00 0.60 3.66 2.00 0.60 3.71 2.00 0.60 3.80 3.00 0.90 3.60 3 0.90 3.62 3 0.90 3.73 6.00 1.80 3.50 6 1.80 3.53 6 1.80 3.60 16.00 4.80 3.33 16 4.80 3.40 16 4.80 3.43 CO2-CH4-H2O
(3∶7)1.30 0.39 3.74 1.30 0.39 3.78 1.30 0.39 3.91 2.00 0.60 3.69 2.00 0.60 3.70 2.00 0.60 3.79 3.00 0.90 3.58 3 0.90 3.62 3 0.90 3.70 6.00 1.80 3.47 6 1.80 3.54 6 1.80 3.60 16.00 4.80 3.41 16 4.80 3.40 16 4.80 3.48 CO2-N2-H2O
(1∶9)3.90 0.39 3.76 3.90 0.39 3.88 3.90 0.39 3.91 6.00 0.60 3.68 6 0.6 3.78 6.0 0.60 3.82 9.00 0.90 3.60 9 0.9 3.69 9 0.90 3.74 18.00 1.80 3.51 18 1.8 3.61 18 1.80 3.63 CO2-CH4-H2O
(1∶9)3.90 0.39 3.78 3.90 0.39 3.84 3.90 0.39 3.93 6.00 0.60 3.71 6 0.6 3.76 6.00 0.60 3.86 9.00 0.90 3.63 9 0.9 3.67 9 0.90 3.77 18.00 1.80 3.56 18 1.8 3.59 18 1.80 3.66 注:反应系统中气体比例为摩尔比,下同 表 3 纯/非纯CO2-水体系的pH预测模型
Table 3. pH prediction models for pure/impure CO2-water systems
化学反应式 数学表达式 气−液平衡: $ {\mathrm{C}\mathrm{O}}_{2\left(\mathrm{g}\right)}+{\mathrm{H}}_{2}\mathrm{O}\leftrightarrow {\mathrm{H}\mathrm{C}\mathrm{O}}_{3}^{-}+{\mathrm{H}}^{+} $ $ {y}_{g}P{\varnothing }_{g}{K}_{g}=\prod\limits_{j=1}^{N_c}{c}_{j}^{{v}_{gj}}{\gamma }_{j}^{{v}_{gj}},g=1,\cdots ,{N}_{g} $
式中:$ {y}_{g} $气体组分在气相中的摩尔浓度;$ {\varnothing }_{g} $为气体逸度系数;$ P $为压力;$ {K}_{g} $为气−液平衡常数;$ {c}_{j} $为主要组分浓度;$ {\gamma }_{j} $为活度系数;$ {v}_{gj} $为气体组分与主要组分的反应系数;$ N_c $,$ {N}_{g} $分别为主要组分和气体组分数目$ {\mathrm{N}}_{2\left(\mathrm{g}\right)}\leftrightarrow {\mathrm{N}}_{2\left(\mathrm{a}\mathrm{q}\right)} $ $ {\mathrm{C}\mathrm{H}}_{4\left(\mathrm{g}\right)}\leftrightarrow {\mathrm{C}\mathrm{H}}_{4\left(\mathrm{a}\mathrm{q}\right)} $ 水溶液中化学组分平衡: $ {\mathrm{C}\mathrm{O}}_{3}^{2-}+{\mathrm{H}}^{+}\leftrightarrow {\mathrm{H}\mathrm{C}\mathrm{O}}_{3}^{-} $ $ {c}_{k}={K}_{k}^{-1}{\gamma }_{k}^{-1}\prod\limits_{j=1}^{N_c}{c}_{j}^{{v}_{kj}}{\gamma }_{j}^{{v}_{kj}},k=1,\cdots ,{N}_{a} $
式中:$ {c}_{k} $为次要组分;$ {K}_{k} $为液相中化学反应平衡常数;$ {v}_{kj} $为次要组分与主要组分的反应系数;$ {N}_{a} $为液相中次要组分数目$ {\mathrm{H}}_{2}\mathrm{O}+{\mathrm{C}\mathrm{O}}_{2\left(\mathrm{a}\mathrm{q}\right)}\leftrightarrow {\mathrm{H}\mathrm{C}\mathrm{O}}_{3}^{-}+{\mathrm{H}}^{+} $ $ {\mathrm{H}}^{+}+{\mathrm{O}\mathrm{H}}^{-}\leftrightarrow {\mathrm{H}}_{2}\mathrm{O} $ -
[1] 谢和平,刘涛,吴一凡,等. CO2的能源化利用技术进展与展望[J]. 工程科学与技术,2022,54(1):145-156.XIE H P,LIU T,WU Y F,et al. Progress and prospect of CO2 energy utilization technology[J]. Advanced Engineering Sciences,2022,54(1):145-156. (in Chinese with English abstract [2] 郭克星,闫光龙,张阿昱,等. CO2捕集、利用与封存技术及CO2管道研究现状与发展[J]. 天然气与石油,2023,41(1):28-40.GUO K X,YAN G L,ZHANG A Y,et al. Status quo and development of the research on CO2 capture,utilization and storage technology and CO2 pipeline[J]. Natural Gas and Oil,2023,41(1):28-40. (in Chinese with English abstract [3] 王延欣. 枯竭油气藏储集库储热供暖耦合CO2封存性能分析[J]. 地质科技通报,2024,43(3):12-21.WANG Y X. Performance analysis of thermal energy storage for space heating and CO2 sequestration in depleted oil and gas reservoirs[J]. Bulletin of Geological Science and Technology,2024,43(3):12-21. (in Chinese with English abstract [4] 赵震宇,姚舜,杨朔鹏,等. “双碳” 目标下:中国CCUS发展现状、存在问题及建议[J]. 环境科学,2023,44(2):1128-1138.ZHAO Z Y,YAO S,YANG S P,et al. Under goals of carbon peaking and carbon neutrality:Status,problems,and suggestions of CCUS in China[J]. Environmental Science,2023,44(2):1128-1138. (in Chinese with English abstract [5] 吴江,任思源,孙一景,等. 基于“双碳” 背景的CCUS技术研究与应用[J]. 华中科技大学学报(自然科学版),2022,50(7):89-100.WU J,REN S Y,SUN Y J,et al. Research and application of CCUS technology based on "double carbon" background[J]. Journal of Huazhong University of Science and Technology (Natural Science Edition),2022,50(7):89-100. (in Chinese with English abstract [6] IEA. Explore energy data by category,indicator,country or region[EB/OL]. https://www.iea.org/data-and-statistics/data-browser/country=WORLD&fuel=CO2%20emissions&indicator=TotCO2. 2021-03-02. [7] 许毛,张贤,樊静丽,等. 我国煤制氢与CCUS技术集成应用的现状、机遇与挑战[J]. 矿业科学学报,2021,6(6):659-666.XU M,ZHANG X,FAN J L,et al. Status quo,opportunities and challenges of integrated application of coal-to-hydrogen and CCUS technology in China[J]. Journal of Mining Science and Technology,2021,6(6):659-666. (in Chinese with English abstract [8] 孙旭东,张蕾欣,张博. 碳中和背景下我国煤炭行业的发展与转型研究[J]. 中国矿业,2021,30(2):1-6.SUN X D,ZHANG L X,ZHANG B. Research on the coal industry development and transition in China under the background of carbon neutrality[J]. China Mining Magazine,2021,30(2):1-6. (in Chinese with English abstract [9] 谢昂均,杨正军,徐钢,等. 我国能源行业实现碳中和的产业链情景分析[J]. 动力工程学报,2024,44(11):1733-1740.XIE A J,YANG Z J,XU G,et al. Scenario analysis of the industrial chain for achieving carbon neutrality in China's energy industry[J]. Journal of Chinese Society of Power Engineering,2024,44(11):1733-1740. (in Chinese with English abstract [10] 罗亚南,蒋坤卿,黄思浩,等. 地热水回灌耦合CO2地质封存系统安全性分析[J]. 地质科技通报,2024,43(3):59-67.LUO Y N,JIANG K Q,HUANG S H,et al. Safety analysis of geothermal water recharge coupled with CO2 geological storage system[J]. Bulletin of Geological Science and Technology,2024,43(3):59-67. (in Chinese with English abstract [11] 郑长远,雷宏武,崔银祥,等. 西宁盆地南部天然CO2泄漏和浅部含水层响应[J]. 地质科技通报,2023,42(6):223-232.ZHENG C Y,LEI H W,CUI Y X,et al. Natural CO2 leakage and responses of shallow aquifers in the southern Xining Basin[J]. Bulletin of Geological Science and Technology,2023,42(6):223-232. (in Chinese with English abstract [12] 严刚,郑逸璇,王雪松,等. 基于重点行业/领域的我国碳排放达峰路径研究[J]. 环境科学研究,2022,35(2):309-319.YAN G,ZHENG Y X,WANG X S,et al. Pathway for carbon dioxide peaking in China based on sectoral analysis[J]. Research of Environmental Sciences,2022,35(2):309-319. (in Chinese with English abstract [13] ZHANG X,FAN J L,WEI Y M. Technology roadmap study on carbon capture,utilization and storage in China[J]. Energy Policy,2013,59:536-550. doi: 10.1016/j.enpol.2013.04.005 [14] 张凯,陈掌星,兰海帆,等. 碳捕集、利用与封存技术的现状及前景[J]. 特种油气藏,2023,30(2):1-9. doi: 10.3969/j.issn.1006-6535.2023.02.001ZHANG K,CHEN Z X,LAN H F,et al. Status and prospects of carbon capture,utilization and storage technology[J]. Special Oil & Gas Reservoirs,2023,30(2):1-9. (in Chinese with English abstract doi: 10.3969/j.issn.1006-6535.2023.02.001 [15] 胡其会,李玉星,张建,等. “双碳” 战略下中国CCUS技术现状及发展建议[J]. 油气储运,2022,41(4):361-371.HU Q H,LI Y X,ZHANG J,et al. Current status and development suggestions of CCUS technology in China under the "Double Carbon" strategy[J]. Oil & Gas Storage and Transportation,2022,41(4):361-371. (in Chinese with English abstract [16] JIANG K,ASHWORTH P,ZHANG S Y,et al. China's carbon capture,utilization and storage (CCUS) policy:A critical review[J]. Renewable and Sustainable Energy Reviews,2020,119:109601. doi: 10.1016/j.rser.2019.109601 [17] 崔国栋,胡哲,宁伏龙,等. 咸水层毛管力圈闭机制及对非纯CO2封存效率的影响[J]. 煤炭学报,2023,48(7):2791-2801.CUI G D,HU Z,NING F L,et al. Local capillary entrapment and its effect on sequestration efficiencies during impure CO2 injection into saline aquifers[J]. Journal of China Coal Society,2023,48(7):2791-2801. (in Chinese with English abstract [18] 杨术刚,蔡明玉,张坤峰,等. CO2−水−岩相互作用对CO2地质封存体物性影响研究进展及展望[J]. 油气地质与采收率,2023,30(6):80-91.YANG S G,CAI M Y,ZHANG K F,et al. Research progress and prospect of CO2-water-rock interaction on petrophysical properties of CO2 geological sequestration[J]. Petroleum Geology and Recovery Efficiency,2023,30(6):80-91. (in Chinese with English abstract [19] 李义曼,庞忠和. 二氧化碳地质封存中的水-岩反应动力学模拟:进展及问题[J]. 吉林大学学报(地球科学版),2012,42(增刊2):352-360.LI Y M,PANG Z H. Development and issue on kinetic model of water-rock interaction in CO2 geological sequestion[J]. Journal of Jilin University (Earth Science Edition),2012,42(S2):352-360. (in Chinese with English abstract [20] 任岚,于志豪,赵金洲,等. 碳酸溶蚀对致密砂岩储层流动特征的影响:以鄂尔多斯盆地长6致密砂岩为例[J]. 大庆石油地质与开发,2023,42(2):50-58.REN L,YU Z H,ZHAO J Z,et al. Impact of carbonic acid dissolution on flow characteristics of tight sandstone reservoir:Taking Chang 6 tight sandstone in Ordos Basin as an example[J]. Petroleum Geology & Oilfield Development in Daqing,2023,42(2):50-58. (in Chinese with English abstract [21] 郭琦,吴欣强,韩恩厚,等. 高温水溶液pH值原位测量系统与机理[J]. 应用化学,2016,33(11):1329-1336.GUO Q,WU X Q,HAN E H,et al. In-situ pH measurement system and mechanism for high temperature aqueous solutions[J]. Chinese Journal of Applied Chemistry,2016,33(11):1329-1336. (in Chinese with English abstract [22] KAKIUCHI T. Salt bridge in electroanalytical chemistry:Past,present,and future[J]. Journal of Solid State Electrochemistry,2011,15(7):1661-1671. [23] SHIBATA M,SAKAIDA H,KAKIUCHI T. Determination of the activity of hydrogen ions in dilute sulfuric acids by use of an ionic liquid salt bridge sandwiched by two hydrogen electrodes[J]. Analytical Chemistry,2011,83(1):164-168. doi: 10.1021/ac1021216 [24] YAMADA A,MOHRI S,NAKAMURA M,et al. A simple method for decreasing the liquid junction potential in a flow-through-type differential pH sensor probe consisting of pH-FETs by exerting spatiotemporal control of the liquid junction[J]. Sensors,2015,15(4):7898-7912. doi: 10.3390/s150407898 [25] MEYSSAMI B,BALABAN M O,TEIXEIRA A A. Prediction of pH in model systems pressurized with carbon dioxide[J]. Biotechnology Progress,1992,8(2):149-154. doi: 10.1021/bp00014a009 [26] TODDSCHAEF H,PETER MCGRAIL B. Direct measurements of pH and dissolved CO2 in H2O-CO2 brine mixtures to supercritical conditions[J]. Greenhouse Gas Control Technologies 7,2005,15(4):2169-2173. [27] PENG C,CRAWSHAW J P,MAITLAND G C,et al. The pH of CO2-saturated water at temperatures between 308 K and 423 K at pressures up to 15 MPa[J]. The Journal of Supercritical Fluids,2013,82:129-137. doi: 10.1016/j.supflu.2013.07.001 [28] TRUCHE L,BAZARKINA E F,BERGER G,et al. Direct measurement of CO2 solubility and pH in NaCl hydrothermal solutions by combining in situ potentiometry and Raman spectroscopy up to 280℃ and 150 bar[J]. Geochimica et Cosmochimica Acta,2016,177:238-253. doi: 10.1016/j.gca.2015.12.033 [29] LI X L,PENG C,CRAWSHAW J P,et al. The pH of CO2-saturated aqueous NaCl and NaHCO3 solutions at temperatures between 308 K and 373 K at pressures up to 15 MPa[J]. Fluid Phase Equilibria,2018,458:253-263. doi: 10.1016/j.fluid.2017.11.023 [30] MUTAILIPU M,LIU Y,SONG Y C,et al. The pH of CO2-saturated aqueous KCl solutions at temperatures between 298 K and 423 K at pressures up to 13.5 MPa[J]. Chemical Engineering Science,2021,234:116434. doi: 10.1016/j.ces.2020.116434 [31] SHAO H B,THOMPSON C J,QAFOKU O,et al. In situ spectrophotometric determination of pH under geologic CO2 sequestration conditions:Method development and application[J]. Environmental Science & Technology,2013,47(1):63-70. [32] HAGHI R K,CHAPOY A,PEIRERA L M C,et al. pH of CO2 saturated water and CO2 saturated brines:Experimental measurements and modelling[J]. International Journal of Greenhouse Gas Control,2017,66:190-203. doi: 10.1016/j.ijggc.2017.10.001 [33] PENG D Y,ROBINSON D B. A new two-constant equation of state[J]. Industrial & Engineering Chemistry Fundamentals,1976,15(1):59-64. [34] PITZER K S. Thermodynamics of electrolytes: I. Theoretical basis and general equations[J]. The Journal of Physical Chemistry,1973,77(2):268-277. doi: 10.1021/j100621a026 [35] WOLERY T J. EQ3NR,a computer program for geochemical aqueous speciation-solubility calculations:Theoretical manual,user's guide,and related documentation (Version 7.0,Part 3) [R]. [S.1]:[s.n.],1992. [36] LI D D,DUAN Z H. The speciation equilibrium coupling with phase equilibrium in the H2O-CO2-NaCl system from 0 to 250℃,from 0 to 1000 bar,and from 0 to 5 molality of NaCl[J]. Chemical Geology,2007,244(3/4):730-751.[37] DUAN Z H,SUN R. An improved model calculating CO2 solubility in pure water and aqueous NaCl solutions from 273 to 533 K and from 0 to 2000 bar[J]. Chemical Geology,2003,193(3/4):257-271.[38] BATES R G,VIJH A K. Determination of pH:Theory and practice[J]. Journal of the Electrochemical Society,1973,120(8):263C. doi: 10.1149/1.2403829 [39] ARNÓRSSON S. Deposition of calcium carbonate minerals from geothermal waters:Theoretical considerations[J]. Geothermics,1989,18(1/2):33-39. [40] LEI H W,LI J,LI X C,et al. EOS7Cm:An improved TOUGH2 module for simulating non-isothermal multiphase and multicomponent flow in CO2-H2S-CH4-brine systems with high pressure,temperature and salinity[J]. Computers & Geosciences,2016,94:150-161. [41] LEI H W,LI J,LI X C,et al. Numerical modeling of co-injection of N2 and O2 with CO2 into aquifers at the Tongliao CCS site[J]. International Journal of Greenhouse Gas Control,2016,54:228-241. doi: 10.1016/j.ijggc.2016.09.010 -
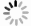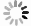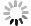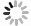




 下载:
下载: